
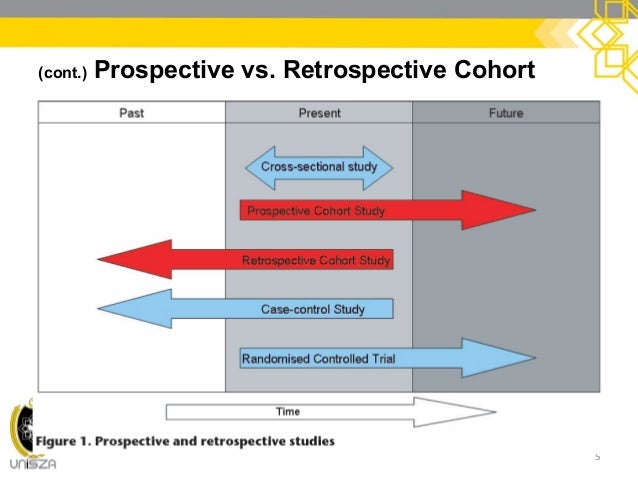
This chapter compares prospective and retrospective observational study design with a discussion of the components, strengths, and weaknesses, while providing examples of each approach. 78: Regulatory Binder and Essential Documents.69: Participant Recruitment and Enrollment.54: Research Questions, Hypotheses, Aims, and Abstract.53: Developing the Idea With Stakeholder Input.47: Statistical Tools for Agreement and Reliability Studies.46: Validity and Performance of Screening: Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value.45: Epidemiology Study: Incidence and Prevalence.43: Sources of Error: Selection Bias, Information Bias, and Confounding.38: Measures of Effect Sizes for Categorical Outcomes.37: Significance Tests: Categorical Data.32: Comparing Independent Samples for Continuous-Type Outcomes: Three Groups or More.31: Comparing Independent Samples With Continuous-Type Outcomes: Two Groups.30: Comparing Matched Samples With Continuous-Type Outcomes: Two Groups.18: Developing and Evaluating Systematic Reviews and Practice Guidelines.17: Recommendations for Reporting Research Studies.12: Special Issues in Randomized Controlled Trials.

10: Choice of Control Groups in Treatment Studies.8: Longitudinal Cohort Versus Cross-Sectional Cohort Studies.5: Observational Studies: Retrospective Versus Prospective.1: Development and Testing of Treatments.


 0 kommentar(er)
0 kommentar(er)
